Abstract:
This article presents a comprehensive safety evaluation of Bifidobacterium animalis subsp. lactis BLa80, a new probiotic strain with potential applications in the food industry. The safety assessment was conducted through in vitro assays simulating gastrointestinal conditions, genomic analysis, and a 90-day oral toxicity test in rats. The strain demonstrated high survival rates under simulated digestive conditions, showed no hemolytic activity, produced no biogenic amines, and exhibited antibiotic susceptibility. Genomic analysis confirmed the absence of virulence factors, and the in vivo toxicity study revealed no adverse effects. Overall, BLa80 is classified as safe for use as a probiotic.
Keywords: Bifidobacterium animalis, probiotics, safety evaluation, toxicity, genomic analysis, gastrointestinal survival.
Introduction
Probiotics have gained attention for their health benefits, particularly in maintaining gut microflora balance and supporting gastrointestinal health. Bifidobacterium animalis subsp. lactis is widely used in the food industry for its probiotic properties. This study evaluates the safety of the newly developed strain BLa80, focusing on its in vitro and in vivo safety profile, genomic characteristics, and overall suitability for consumption.
Materials and Methods
l Strain and Culture
Bifidobacterium animalis subsp. lactis BLa80 was isolated from breast milk and cultured in MRS medium.
l In Vitro Gastrointestinal Simulation
BLa80 was exposed to artificial gastric fluid at pH 2.5 and intestinal fluid at pH 8.0 to evaluate its survival under simulated human digestive conditions. The survival rate was measured after 2 hours of incubation.
l Hemolysis Test
Hemolytic activity was tested on blood agar plates to assess any potential pathogenicity. Staphylococcus aureus was used as a positive control for β-hemolysis.
l Antibiotic Susceptibility
Antibiotic susceptibility of BLa80 was tested against a panel of antibiotics, including ampicillin, clindamycin, and chloramphenicol, following Clinical Laboratory Standards Institute (CLSI) guidelines.
l Biogenic Amine Production
High-performance liquid chromatography (HPLC) was used to test for the production of biogenic amines like histamine and tyramine.
l Bile Salt Hydrolase Activity
The strain’s ability to hydrolyze bile salts was tested by cultivating BLa80 on bile salt-supplemented media.
l Genomic Analysis
Whole-genome sequencing was performed to assess the presence of virulence factors, antibiotic resistance genes, and metabolic pathways.
l 90-Day Oral Toxicity Test
Rats were administered BLa80 at varying doses for 90 days. The study monitored toxicity, including body weight changes, food consumption, and organ health, to determine the No Observed Adverse Effect Level (NOAEL).
l Bacterial Translocation Test
A translocation assessment was conducted to check for the movement of BLa80 to other organs, including the heart, liver, and kidneys, after ingestion.
Results
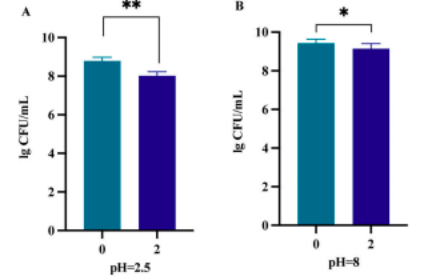
l Survival in Simulated Digestive Fluids
BLa80 exhibited a 99.92% survival rate in artificial intestinal fluid and 91.14% in gastric fluid, indicating strong resilience to digestive conditions.
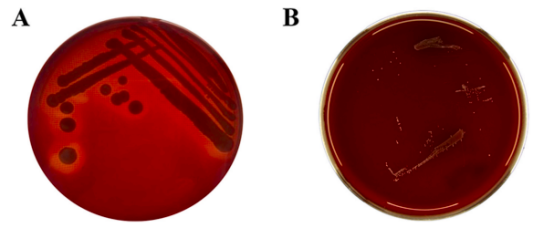
l Absence of Hemolytic Activity
No hemolysis was observed in BLa80 cultures(B), confirming its non-pathogenic nature.
l Antibiotic Sensitivity
BLa80 was sensitive to all antibiotics tested, with MIC values below resistance thresholds.
l No Biogenic Amine Production
None of the harmful biogenic amines, such as histamine or tyramine, were detected in the strain.
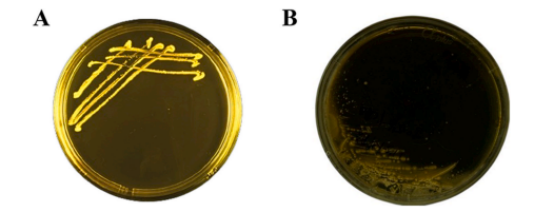
l Bile Salt Hydrolase Activity
BLa80 demonstrated bile salt hydrolase activity, which is beneficial for gut survival and function(A).
l Genomic Safety
Genomic sequencing confirmed that BLa80 does not carry any virulence or antibiotic resistance genes, further supporting its safety.
l Oral Toxicity Results
The 90-day oral toxicity test showed no adverse effects in rats, confirming a NOAEL of 1.76 g/kg for female rats and 1.64 g/kg for male rats.
l Bacterial Translocation
No translocation of BLa80 was detected in any of the tested organs, indicating that the strain does not migrate beyond the gastrointestinal tract.
Discussion
The safety profile of Bifidobacterium animalis subsp. lactis BLa80 is consistent with established standards for probiotic strains. Its high survival rate in gastrointestinal conditions, lack of hemolysis, and sensitivity to antibiotics all support its classification as safe. Furthermore, the absence of biogenic amine production and translocation, combined with the 90-day toxicity test, provides a robust basis for its use in food products.
Conclusion
The safety assessment of Bifidobacterium animalis subsp. lactis BLa80 demonstrates that it is suitable for use as a probiotic in the food industry. Further studies can focus on its efficacy and potential health benefits in human clinical trials.
References
· Hill, C., Guarner, F., Reid, G., et al. (2014). "Expert consensus on probiotics." Nat Rev Gastroenterol Hepatol 11(8): 506–514.
· Zhou, J., Shu, Q., Rutherfurd, K., et al. (2000). "Acute oral toxicity and bacterial translocation studies on probiotic strains." Food Chem Toxicol 38(2-3): 153–161.









