Effects of Acacia Fiber and Probiotic Bifidobacterium Lactis (BLa80) on Gastrointestinal Complaints in Constipation-Predominant Irritable Bowel Syndrome: A Randomized Double-Blind Placebo-Controlled Trial
Authors: Lonneke Janssen Duijghuijsen, Maartje van den Belt, Iris Rijnaarts, Paul Vos, Damien Guillemet, Ben Witteman, Nicole de Wit
Journal: European Journal of Nutrition
DOI: [10.1007/s00394-024-03398-8](https://doi.org/10.1007/s00394-024-03398-8)
Abstract:
This study aimed to determine the effects of a 4-week intervention with either Acacia fiber (AF) with prebiotic properties or a probiotic Bifidobacterium Lactis (BLa80) supplement compared to a placebo on stool pattern, IBS symptoms, and Quality of Life (QoL) in individuals with constipation-predominant IBS (IBS-C). The randomized double-blind placebo-controlled trial included 180 participants meeting the ROME IV criteria for IBS-C. Results showed significant improvements in stool frequency for both AF and BLa80 groups compared to placebo. Probiotic BLa80 also significantly reduced IBS symptom severity, although no significant changes in stool consistency, stool mass, or QoL measures were observed between treatment groups.
Introduction:
Irritable Bowel Syndrome (IBS) is a prevalent functional gastrointestinal disorder affecting 10-20% of the global population, with IBS-C being a common subtype characterized by constipation symptoms such as straining, hard stools, and infrequent bowel movements. Current treatments are inadequate, and dietary interventions, particularly involving prebiotics and probiotics, show potential for symptom relief.
Methods:
- Study Design: Parallel, double-blind, randomized controlled trial.
- Participants: 180 subjects with IBS-C, aged 18-70, BMI 18.5-30 kg/m².
- Intervention: Subjects received either AF (10 g/day), Probiotic BLa80 (4 g/day; 2 × 10¹¹ CFU/g), or a maltodextrin placebo (10 g/day) for 4 weeks following a 4-week observation period.
- Outcomes Measured: Stool frequency, stool consistency, stool mass, IBS severity, constipation symptoms, anxiety and depression scores, and QoL. Daily questionnaires and stool mass collection were used for data gathering.
Results:
- Primary Outcomes: Both AF and Probiotic BLa80 significantly improved stool frequency compared to placebo (AF: P 0.001, BLa80: P = 0.02).
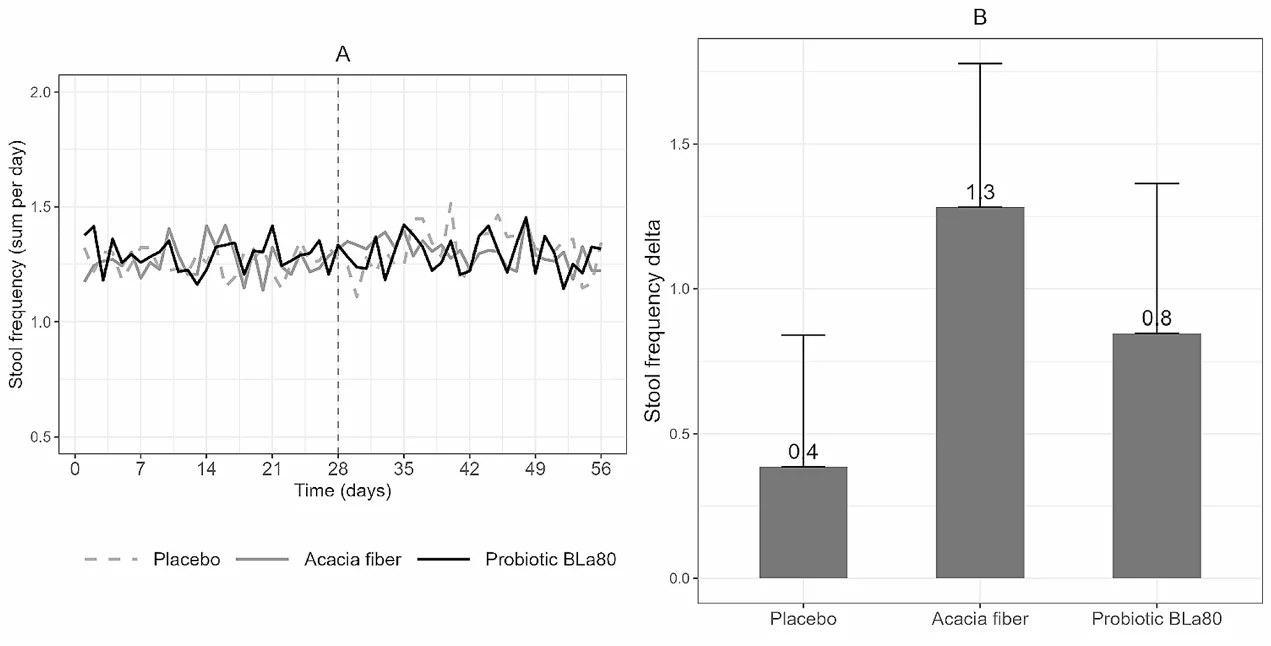
Daily variation in stool frequency and change in stool frequency per week
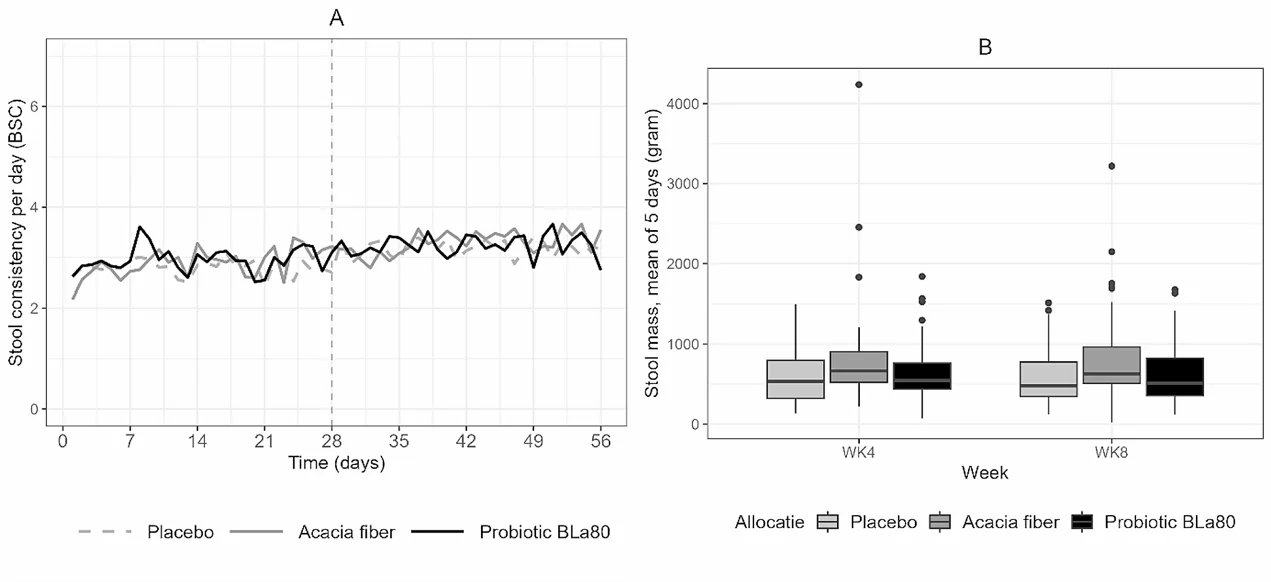
Stool consistency and stool mass changes during the observation and intervention period
- Secondary Outcomes: Probiotic BLa80 reduced IBS symptom severity (P = 0.03). A trend towards decreased constipation symptoms was observed with AF (P = 0.10). No significant differences in stool consistency, stool mass, or QoL measures were noted between the groups.
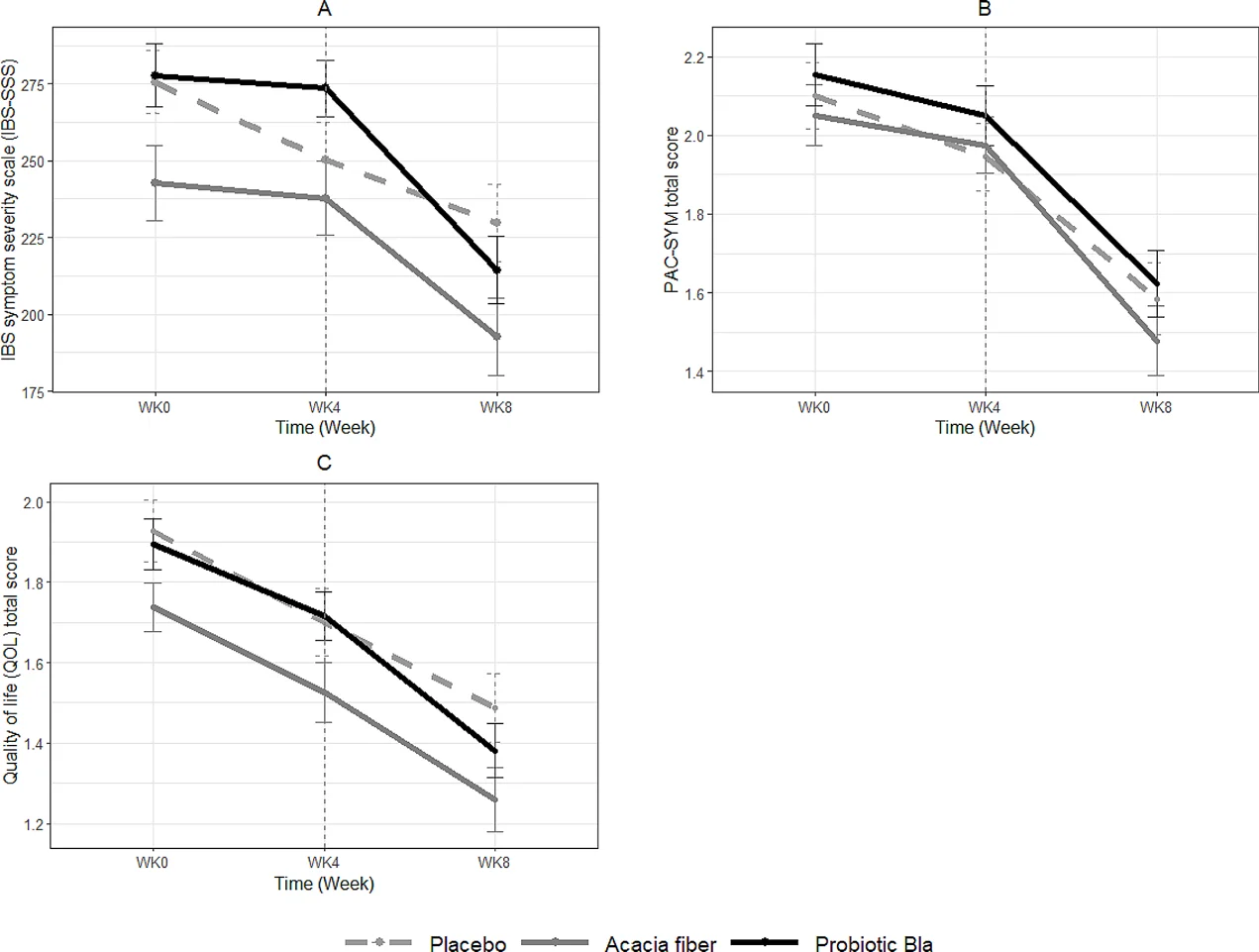
IBS symptom severity scores (IBS-SSS) (A), constipation-related complaints (B), and Quality of Life (QoL) scores (C) during the observation and intervention period
Discussion:
The results suggest that daily supplementation with Acacia fiber and probiotic BLa80 can help alleviate IBS-C related complaints by improving stool frequency and reducing symptom severity, respectively. However, no significant impact was observed on stool consistency, stool mass, or overall quality of life. Further research is needed to explore the long-term benefits and underlying mechanisms of these dietary interventions.
Conclusion:
Dietary supplementation with Acacia fiber and probiotic BLa80 presents a promising approach to managing IBS-C symptoms, particularly in improving stool frequency and reducing overall IBS symptom severity. These findings contribute to the growing body of evidence supporting the use of specific dietary interventions in the management of functional gastrointestinal disorders.
Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT04798417
Keywords: Irritable Bowel Syndrome, Constipation, Fiber, Probiotics, Stool Frequency, IBS Symptoms







